Once the probiotic strains have been selected based on criteria of gastro-resistance, adhesion to the intestinal mucosa and sustainability/stability over time (see Selecting probiotic strains: the HMQ charter), they are subject to studies that validate their clinical usefulness and the doses required for them to act.
These studies are carried out by the PiLeJe research teams in collaboration with other laboratories and research bodies (for instance, Inserm and the Institut Pasteur in Lille).
Tests in vitro and in vivo
The in vitro tests are the first stage in selecting the probiotic strains for a particular indication.
They are used, for instance, to select probiotic strains that can limit the growth of the pathogenic bacteria responsible for diarrhoea (Figure 1) or that can stimulate the production of anti-inflammatory mediators for people with dysbiosis (Figure 2).
Figure 1
Goal:
Inhibition of growth by different strains of two pathogens commonly involved in travelers' diarrhea: Salmonella typhimurium and Escherichia coli
Results:
These 3 probiotic strains are capable of reducing by 80%, two of the bacteria responsible for traveler's diarrhea.
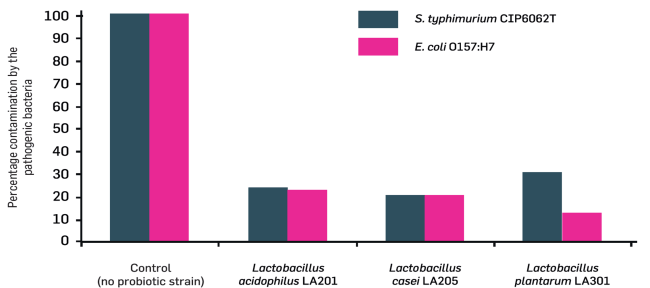
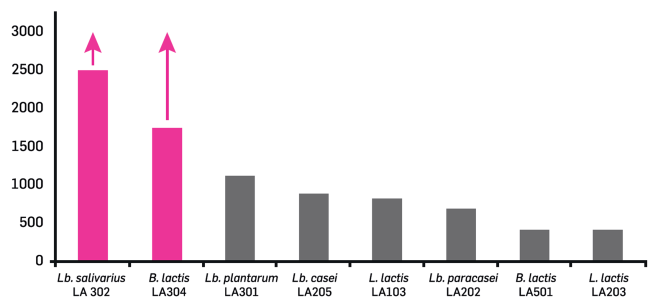
Figure 2
Goal:
The ability of some strains to stimulate the production of Treg-like cytokines (IL-10).
Results:
The probiotic strains Lactobacillus salvarius LA302 and Bifidobacterium lactis LA304 are able to stimulate the production of the anti-inflammatory cytokine IL-10.
Following promising in vitro tests, studies are carried out on laboratory animals: these are in vivo studies.
They are used, for example, to test a mixture of selected probiotic strains on animals with inflammatory colitis or infected with candida albicans.
These studies are published internationally in specialist scientific journals such as the World Journal of Gastroenterology.
Clinical studies
The PiLeJe laboratory performs clinical and observational studies on the products it markets.
They are carried out in partnership with university hospitals, units of the INRA (French National Institute for Agricultural Research) and units of the CNRS (French National Centre for Scientific Research), and are used to collect data on the clinical effectiveness of the PiLeJe health solutions.
A double-blind clinical study* of 100 patients with irritable bowel syndrome (defined according to the Rome criteria), showed that a mixture of 4 probiotic strains selected to relieve the patients’ constipation and abdominal pain was more effective than a placebo.
* Bibliographic reference: “A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome”, Gastroentérologie Clinique et Biologique, March 2008, Vol.32,147-152. S Drouault-Holowacz, S Bieuvelet, A Burckel, M Cazaubien, X Dray, P Marteau

- Alard J, Peucelle V, Boutillier D, Breton J, Kuylle S, Pot B, Holowacz S , Grangette C. Probiotic strains with a high potential for inflammatory bowel disease management identified by combining in vitro and in vivo approaches. Beneficial Microbes 2018, Vol. 9, No. 2, pp. 317-331
- Holowacz S, Guinobert I, Guilbot A, Hidalgo S, Bisson JF. A mixture of five bacterial strains attenuates skin inflammation in mice. Antiinflamm Antiallergy Agents Med Chem. 2018 Aug 13.
- Holowacz S, Blondeau C, Guinobert I, Guilbot A, Hidalgo S, Bisson JF. Lactobacillus salivarius LA307 and Lactobacillus rhamnosus LA305 attenuate skin inflammation in mice. Lactobacillus salivarius LA307 and Lactobacillus rhamnosus LA305 attenuate skin inflammation in mice. Beneficial Microbes 2018, Vol. 9, No. 2, pp. 299-309
- Van Hul M, Geurts L, Plovier H, Druart C, Everard A, Ståhlman M, Rhimi M, Chira K, Teissedre PL, Delzenne NM, Maguin E, Guilbot A, Brochot A, Gerard P, Bäckhed F, Cani PD. Reduced obesity, diabetes and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Endocrinol Metab. 2017 Sep 5:ajpendo.00107.2017. doi:10.1152/ajpendo.00107.2017
- Neau E, Delannoy J, Marion C, Cottart CH, Labellie C, Holowacz S, Butel MJ, Kapel N, Waligora-Dupriet AJ.Three Novel Candidate Probiotic Strains with Prophylactic Properties in a Murine Model of Cow's Milk Allergy. Appl Environ Microbiol. 2016;82(6):1722-33. doi: 10.1128/AEM.03440-15.
- Holowacz S, Blondeau C, Guinobert I, Guilbot A, Hidalgo-Lucas S, Bisson JF. (2016) Antidiarrheal and Antinociceptive Effects of a Probiotic Mixture in Rats. J Prob Health 4: 155. doi: 10.4172/2329-8901.1000155
- Holowacz S, Guigné C, Chêne G, Mouysset S, Guilbot A, Seyrig C, Dubourdeau M. A multispecies Lactobacillus- and Bifidobacterium-containing probiotic mixture attenuates body weight gain and insulin resistance after a short-term challenge with a high-fat diet in C57/BL6J mice. PharmaNutrition. 2015 Jul;3(3):101-7.
- Nébot-Vivinus M, Harkat C, Bzioueche H, Cartier C, Plichon-Dainese R, Moussa L, Eutamene H, Pishvaie D, Holowacz S, Seyrig C, Piche T, Theodorou V. Multispecies probiotic protects gut barrier function in experimental models. World J Gastroenterol. 2014 Jun 14;20(22):6832-43.
- Drouault-Holowacz S, Bieuvelet S, Burckel A, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32(2):147-52.
- Drouault-Holowacz S, Foligné B, Dennin V, Goudercourt D, Terpend K, Burckel A, Pot B. Anti-inflammatory potential of the probiotic dietary supplement Lactibiane Tolérance: in vitro and in vivo considerations. Clin Nutr. 2006;25(6):994-1003.
